Over-the-counter birth management? Drugmaker seeks Food and drug administration acceptance
 [ad_1]
[ad_1]
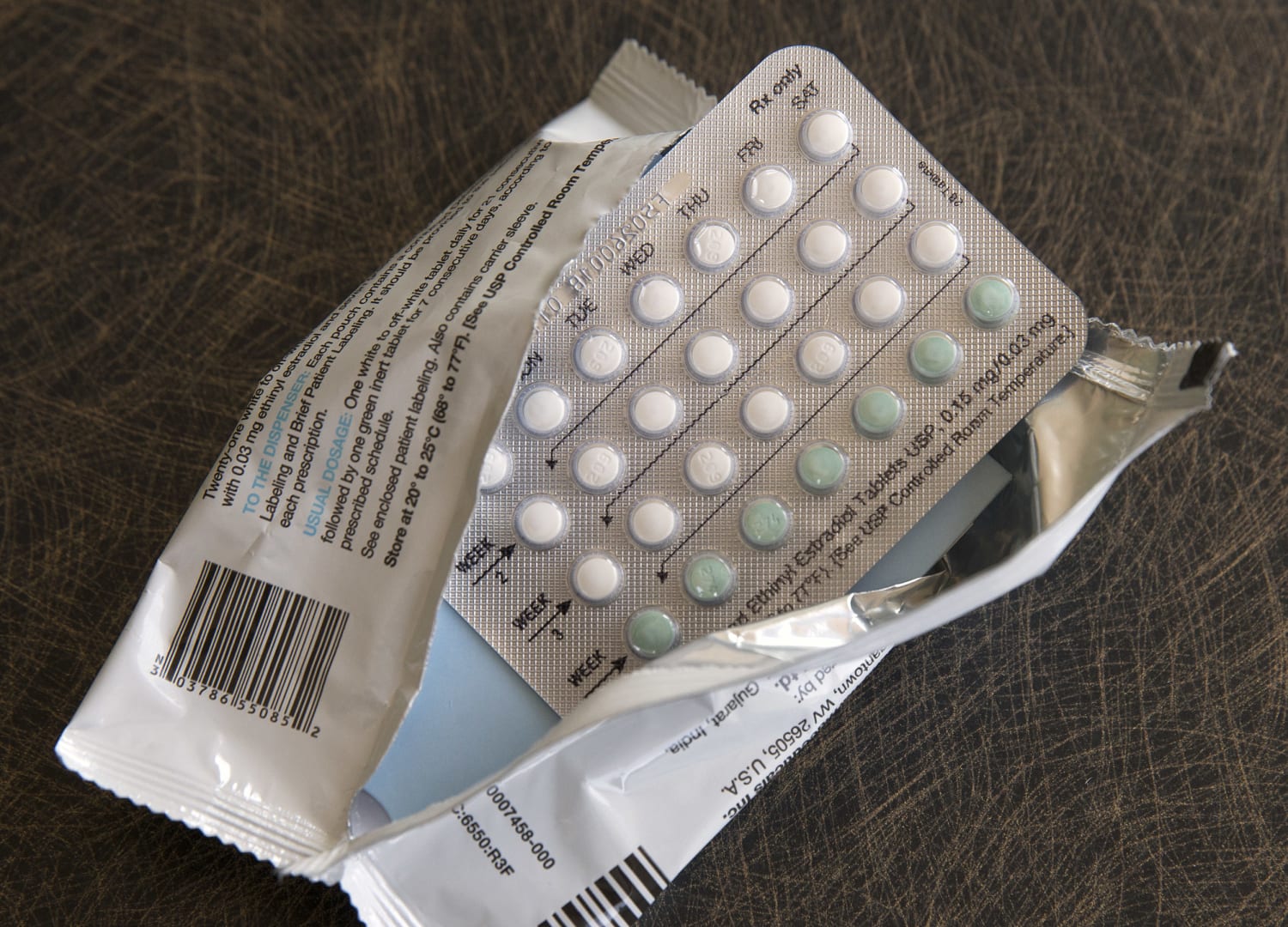
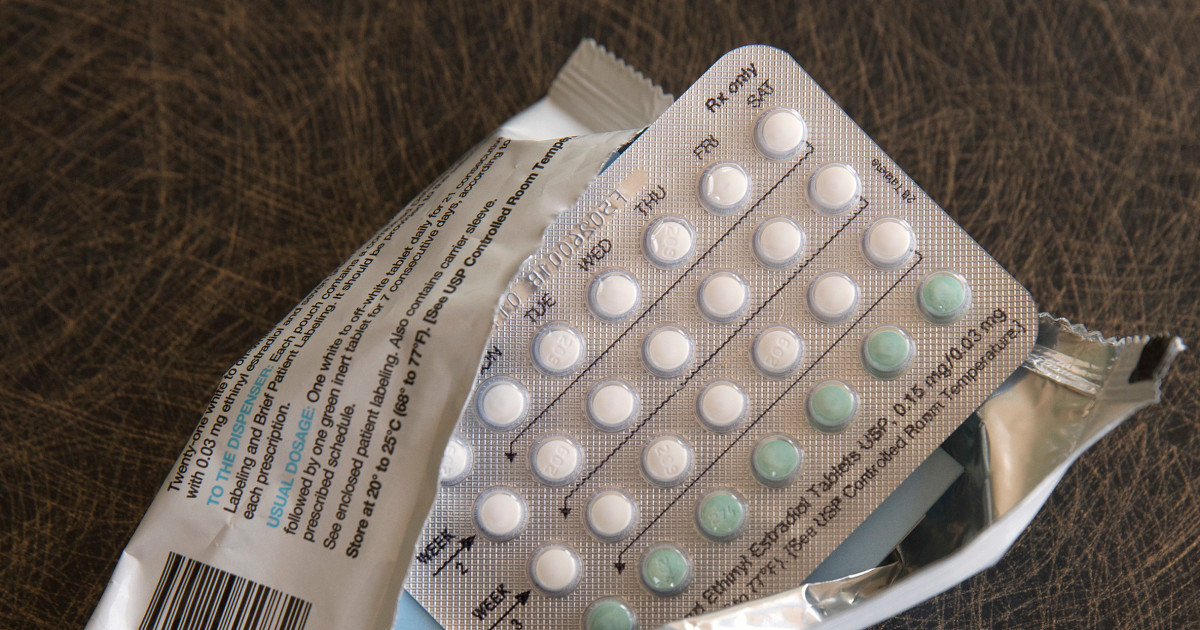
WASHINGTON — For the initial time, a pharmaceutical firm has asked for permission to sell a beginning control pill over the counter in the U.S.
HRA Pharma’s software on Monday sets up a high-stakes conclusion for health regulators amid legal and political battles over women’s reproductive health. The company says the timing was unrelated to the Supreme Court’s recent decision overturning Roe v. Wade.
Hormone-based tablets have prolonged been the most popular sort of beginning regulate in the U.S., utilised by thousands and thousands of females considering that the 1960s. They have often expected a prescription, frequently so health experts can screen for ailments that elevate the chance of scarce, but unsafe, blood clots.
The French drugmaker’s software compiles a long time of research supposed to persuade the Food stuff and Drug Administration that women can properly display screen themselves for individuals hazards and use the pill effectively.
“For a products that has been available for the last 50 yrs, that has been employed safely and securely by thousands and thousands of girls, we believed it was time to make it far more accessible,” stated Frederique Welgryn, HRA’s chief strategy officer.
An Fda approval could come future yr and would only utilize to HRA’s capsule, which would be marketed underneath its primary model identify, Opill. The company obtained the many years-old drug from Pfizer in 2014, but it is not presently promoted in the U.S.
Reproductive rights advocates want to see other prescription contraceptives transfer about the counter and, at some point, for abortion pills to do the same.
That possible for a precedent-environment conclusion when again spots the Food and drug administration below an extreme political highlight.
Late previous year, the agency was condemned by abortion opponents and praised by women’s legal rights advocates when it loosened access to abortion tablets. The agency faced comparable political pressures in 2006 when it accepted above-the-counter use of the unexpected emergency contraception pill Plan B.
Several conservative teams strain they are only intrigued in curtailing abortion, and point out bans frequently explicitly exclude contraception.
Even right before Monday’s announcement, Democratic lawmakers ended up contacting on the Fda to swiftly look at any this kind of requests.
“We urge Fda to evaluate programs for above-the-counter birth management pills with out delay and primarily based solely on the facts,” reported a lot more than 50 customers of the House’s Pro-Preference Caucus in a March letter.
Many frequent drugs have manufactured the change from behind the pharmacy counter, like drugs for suffering relief, heartburn and allergic reactions.
In each and every circumstance, businesses must clearly show that people can comprehend the drug’s labeling, assess its pitfalls and use it securely and properly without the need of specialist supervision. HRA expended seven many years conducting the Food and drug administration-expected scientific tests, including a trial that followed 1,000 women of all ages using its capsule for six months.
At the rear of the company’s endeavours is a coalition of women’s health researchers and advocates who have labored for just about two a long time to make contraceptives a lot more available, primarily to teams with considerably less access to health treatment.
The Oral Contraceptives Around-the-Counter Doing work Team assisted fund some of HRA’s study and is mobilizing help at the rear of a media marketing campaign dubbed No cost the Capsule.
“A great deal of our study has been about earning the situation to help encourage and help a enterprise to take this do the job on,” stated Kelly Blanchard, president of Ibis Reproductive Health, a team member that supports abortion and contraceptive entry.
Start manage capsules are obtainable with out a prescription throughout substantially of South The united states, Asia and Africa. Previous yr, Paris-centered HRA received U.K. approval for the to start with beginning regulate capsule accessible there without having a prescription.
Advocates have been notably intrigued in HRA’s drug since they say it’s most likely to increase much less protection worries.
The tablet includes a single synthetic hormone, progestin, which stops pregnancy by blocking sperm from the cervix.
Most start control drugs have progestin in addition estrogen, which can enable make durations lighter and much more frequent. Progestin-only supplements are generally encouraged for women of all ages who just can't consider the additional well-known combination tablets due to health problems.
But estrogen also accounts for most of the blood clot threat associated with oral contraceptives. FDA’s labeling warns from their use in specified gals now at threat for coronary heart complications, these as these who smoke and are above 35.
For most women of all ages, the medicines are overwhelmingly protected. For every single 10,000 ladies taking combination pills annually, a few to nine will put up with a blood clot, in accordance to Food and drug administration info. That compares with 1 to 5 clots amongst 10,000 women of all ages who are not using birth regulate.
And medical gurus place out that blood clot costs are much greater in gals who become expecting, when hormone ranges and minimized blood flow enhance clotting chance.
“What I surely see is a misunderstanding of the potential risks of these capsules. It is a great deal safer to take the capsule than to be pregnant” reported Dr. Maura Quinlan, a Northwestern College medical doctor and member of the American School of Obstetricians and Gynecologists. She was not associated in HRA’s software or investigation.
The medical affiliation supports making all hormone-based contraceptives out there around the counter. Previous month, the nation’s most significant medical doctor teams, the American Clinical Affiliation, endorsed building birth handle supplements accessible over the counter.
Continue to, guidance is not common.
Diana Zuckerman of the nonprofit Nationwide Heart for Health Investigation claims comparing the safety hazards of the capsules with pregnancy is not the proper method.
Many women consider delivery manage tablets to regulate their intervals or minimize bleeding, explained Zuckerman, whose team evaluates medical investigate. “Those are genuine gains, but they are not really worth the possibility of perhaps fatal blood clots,” she reported.
The Fda has extended monitored the safety of oral contraceptives, updating their warnings about the yrs.
Final yr, the agency positioned a keep on a review by drugmaker Cadence Health, which has also been doing work on an about-the-counter pill. The agency instructed the firm to perform supplemental blood stress checks of trial contributors. The firm claims it is “working to triumph over this regulatory hurdle.”
The Food and drug administration is needed to hold a general public conference to evaluate HRA’s software just before producing a conclusion. Protection concerns are probable to take center phase.
Executives at HRA, which is owned by Perrigo Co., anticipate a conclusion in the to start with 50 % of 2023.
Advocates hope it will be the to start with of lots of.
“Once we see the approval of this product or service, it will exhibit that it’s probable and that the info is robust,” Blanchard said. “Hopefully we’ll see the system pace up from right here.”
[ad_2] https://g3box.org/news/health/over-the-counter-birth-management-drugmaker-seeks-food-and-drug-administration-acceptance/?feed_id=2132&_unique_id=62cc1fe3b15db

0 comments:
Post a Comment