Fda authorizes Pfizer's and Moderna's up to date Covid booster shots
 [ad_1]
[ad_1]
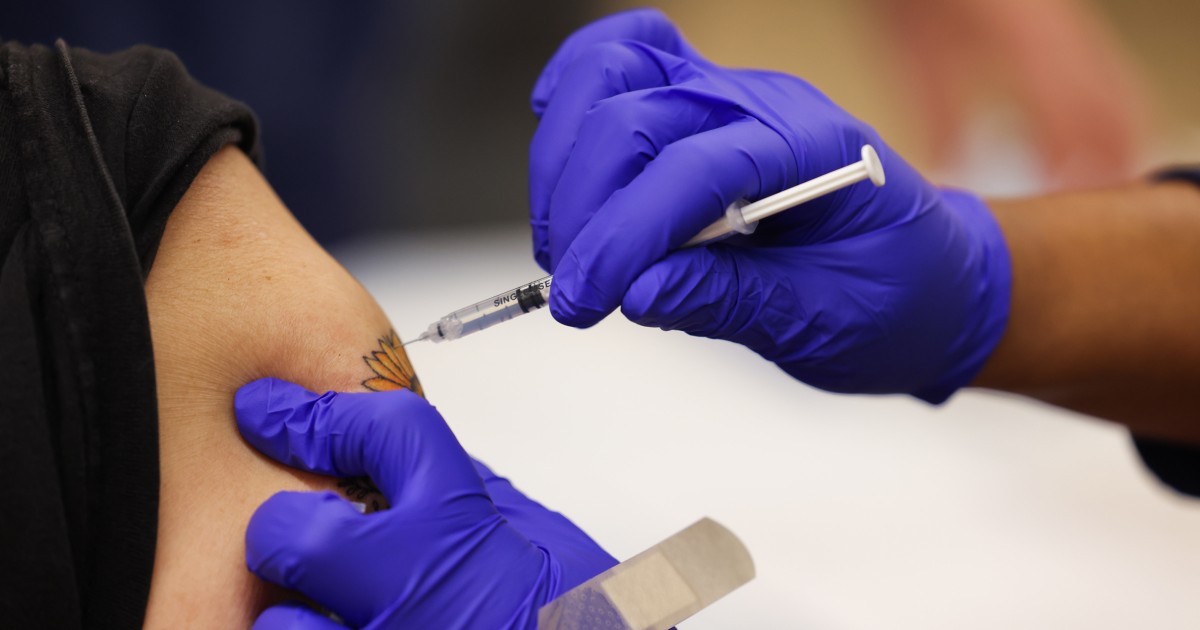
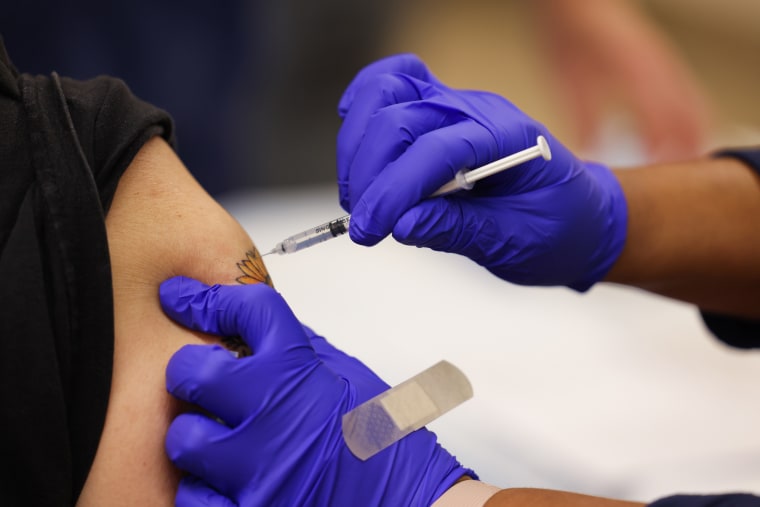
The Food stuff and Drug Administration on Wednesday authorized up to date variations of Pfizer-BioNTech’s and Moderna’s Covid booster shots that goal the really contagious BA.5 omicron subvariant.
The Food and drug administration approved Pfizer’s modified booster for persons ages 12 and more mature Moderna's shot was approved for all those 18 and up.
People who’ve received the two-dose most important series of either vaccine and those who’ve obtained the first two doses plus just one or two boosters are qualified for the updated photographs as extended as two months have passed due to the fact their final shot, the company said in a assertion.
Complete coverage of the Covid-19 pandemic
"The community can be assured that a terrific offer of treatment has been taken by the Fda to guarantee that these bivalent Covid-19 vaccines satisfy our demanding security, effectiveness and production good quality standards for crisis use authorization," Dr. Peter Marks, the agency's prime vaccine regulator, claimed in a assertion.
The FDA’s signoff is not the very last step: The decision will now go to the Facilities for Disease Management and Prevention and its advisory committee to concern their personal suggestion on how the photographs need to be utilised. The agency's Advisory Committee on Immunization Procedures is scheduled to vote Thursday. CDC Director Dr. Rochelle Walensky could indicator off on the doses shortly right after Thursday's meeting, and vaccinations could get started extensively after Labor Day.
The U.S. federal government agreed to buy 105 million doses of Pfizer’s vaccine and 66 million doses of Moderna’s vaccine.
Both modified boosters goal the BA.4 and BA.5 omicron subvariants, in addition to the first coronavirus pressure, in a one shot.
The Fda convened its exterior advisory committee in June to evaluation details on a distinct edition of a booster — just one that merged the original pressure with an earlier variation of omicron, termed BA.1. At the time, the committee voted in favor of updating the shot to focus on omicron, but it did not specify which distinct subvariant.
The Food and drug administration did not consult its advisory committee once again prior to Wednesday's authorizations.
The Biden administration is preparing to distribute the up-to-date shots to young people and older people as part of its tumble booster campaign. The hope, officers have mentioned, is that modifying the vaccines to better match circulating strains will improve the shots’ success and probably deliver more time-lasting immunity.
BA.5 helps make up just about 90% of all new Covid situations in the United States, according to the CDC.
Even now, some exterior researchers say the federal governing administration may be dashing out the new vaccines as well shortly.
The modified boosters have been examined in animal reports, but scientific studies in persons have nonetheless to be concluded. The problem, some say, is that human research may well come across that the new vaccines are no improved than the existing pictures, likely lowering community have faith in in the nation's vaccine marketing campaign.
“Where is the information that implies it is any far better than giving just a strengthen with the ancestral strain?” claimed Dr. Paul Offit, a vaccine professional at the Children’s Medical center of Philadelphia and a member of the FDA's vaccine advisory committee.
The authorities has presently struggled to persuade People to get their initial booster dose. Considerably less than half of People in america who are qualified to get their to start with booster dose have acquired one particular, according to the CDC.
However, other researchers say it “makes sense” to update the vaccines to match the dominant strains. The course of action is related to the flu vaccine, when experts, each and every year, find and take a look at what strain or strains need to be involved in the shot.
“These vaccines will most likely offer much better, but not great, protection versus an infection with omicron and will offer improved defense from symptomatic ailment,” said Dr. Anna Durbin, a vaccine researcher at Johns Hopkins University in Baltimore.
Follow G3 Box News HEALTH on Twitter & Facebook.
[ad_2] https://g3box.org/news/health/fda-authorizes-pfizers-and-modernas-up-to-date-covid-booster-shots/?feed_id=7866&_unique_id=630f80edcc455

0 comments:
Post a Comment